Which Compound in Each Pair Is More Soluble in Water
Which ions are insoluble. Lewiss proposal gives an explanation to the.

If You Love Football You Will Love Aldenham Book A Tour Today Link In Bio Madeinaldenham Aldenhamschoo Aldenham School Education For All Educatio
It can be helpful to write out the empirical formula so you can identify the ions that make up the compound.
. This allows the ionic compound to dissolve whereby water molecules and ions intermingle and mix thoroughly. All of the amines can form hydrogen bonds with water - even the tertiary ones. A Lewis base or electron-pair donor is a molecule with one or more high-energy lone pairs of electrons which can be shared with a low-energy vacant orbital in an acceptor molecule to form an adduct.
Water molecules can attract most ions very well luring the ions away from each other. Ozone has a half-life in pure distilled water of approximately 40 min at pH 76 but this decreases to 10 min at pH 85 Stumm 1958 at 146C. The solubility of each compound in water and hexane will be tested.
This is because chlorine Cl is more electronegative in nature as compared to hydrogen. Look up each ion in. It has a role as a vasodilator agent a bronchodilator agent a muscle relaxant an EC 314 phosphoric diester hydrolase inhibitor an anti-asthmatic drug an anti-inflammatory agent an immunomodulator an.
Covalent compounds are soluble in organic solvents but insoluble in water. Although the tertiary amines dont have a. Lewis proposed an alternative theory of acidbase reactions.
Theophylline is a dimethylxanthine having the two methyl groups located at positions 1 and 3. Benfotiamine S-benzoylthiamine O-monophosphate an amphiphilic S-acyl thiamine derivative prevents the progression of diabetic complications probably by increasing tissue levels of thiamine. An example is the hydrogen chloride molecule HCl.
The reactivity of each compound with a strong acid 3 M HClaq and a strong base 3 M NaOHaq will be determined. The small amines of all types are very soluble in water. How to Use Solubility Rules.
Which ions are slightly soluble. The aldehyde and two ketones will also be tested using Benedicts and Tollens tests for oxidizability. It is approximately 13 times more soluble in water than is oxygen.
At saturation in water at 20C a 2 weight mixture of ozone and oxygen contains about 11 mg of ozone and 40 mg of oxygen per liter. The Lewis theory is based on electronic structureA Lewis base is defined as a compound that can donate an electron pair to a Lewis acid a compound that can accept an electron pair. In the same year that Brønsted and Lowry published their theory G.
It is structurally similar to caffeine and is found in green and black tea. The bonding between hydrogen and chlorine leans more towards chlorine atoms. Identify the compound whose solubility you want to check.
In water the electrostatic forces of attraction between oppositely charged ions are overcome allowing the ions to dissociate and dissolve. Lipid-soluble thiamine precursors have a much higher bioavailability than genuine thiamine and therefore are more suitable for therapeutic purposes. A compound that is soluble in water forms an aqueous solution.
In fact the ones that would normally be found as gases at room temperature are normally sold as solutions in water - in much the same way that ammonia is usually supplied as ammonia solution. In addition to H possible electron-pair acceptors Lewis acids include neutral molecules such as BF 3 and high oxidation state metal ions such as Ag 2 Fe 3 and Mn 7. Which ions are soluble.
In addition you will look up literature values for the melting point and boiling point.

Niacinamide Perfect Skin Care Routine Beauty Skin Care Routine Health Skin Care

Www Facebook Com Doodleopus Andygrayartist Drawing Picnic Basket

Sashiko Style Visible Mending Supply Kit In 2021 Sashiko Visible Mending Sashiko Pattern

Crafters Companion Photopolymer Stamp Have A Wonderful Day Crafters Companion Crafters Stamp

Pin By Christine Tilbrook On Sewing In 2022 Sewing Words Word Search

Answered Activity 1 Strength Of Imfa And Bartleby

Flores Cross Stitch Rose Cross Stitch Flowers Stitch

Answered Activity 1 Factors Affecting The Bartleby

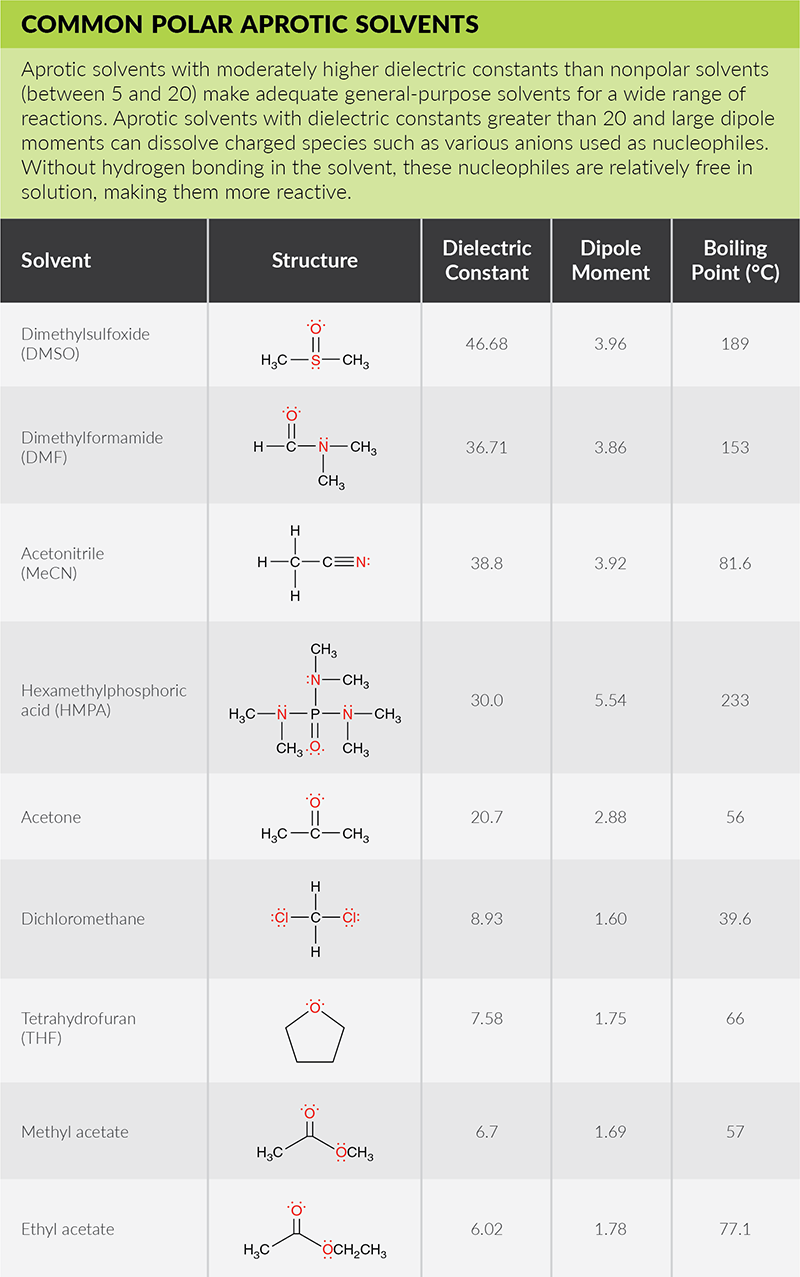
Solubility Factors When Choosing A Solvent News Announcements Cayman Chemical
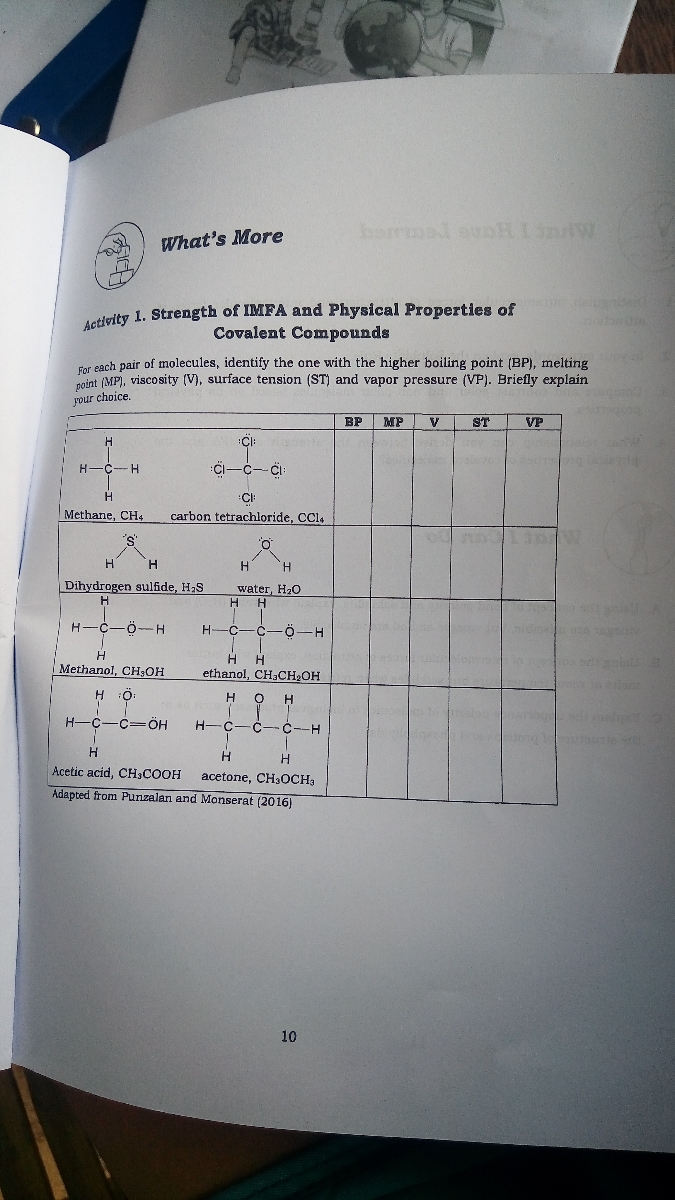
Answered Borrmad Sunhi Sw What S More Activity Bartleby

Peptide Vs Steroid Hormones Steroid Hormone Hormones Peptides

Amongst The Following Compounds Identify Which Are Insoluble Partially Soluble And Highly Soluble In Water I Phenol Ii Toluene Iii Formic Acid Iv Ethylene Glycol V Chloroform Vi Pentanol

Riky Sugar Body Wax 2 22oz Water Solvable Nwt In 2022 Sugar Body Body Waxing Avon Skin So Soft

Covalent Bonds Covalent Chemical Bonds Involve The Sharing Of A Pair Of Valence Electrons By Two At Teaching Chemistry Science Chemistry Chemistry Education
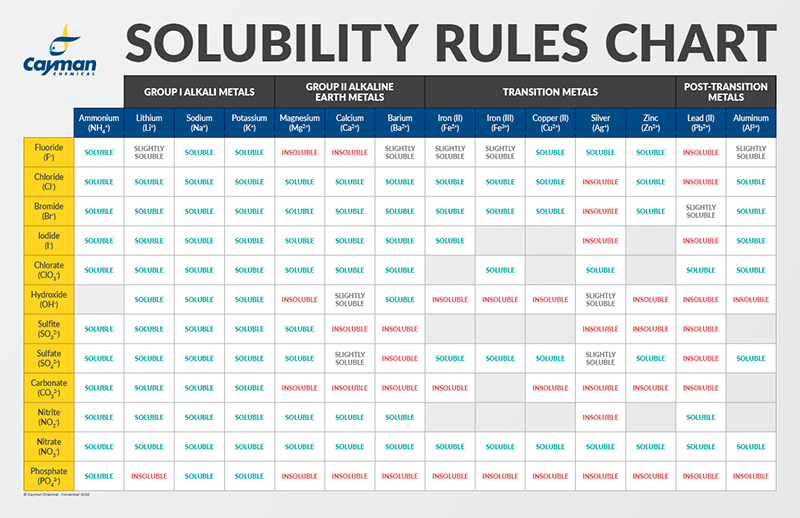
Solubility Factors When Choosing A Solvent News Announcements Cayman Chemical

Can You Mix Powerful Skin Care Ingredients Like Retinol Vitamin C And Ahas Bhas Together Read More Skincare Ingredients Dermatology Skin Care Skin Care


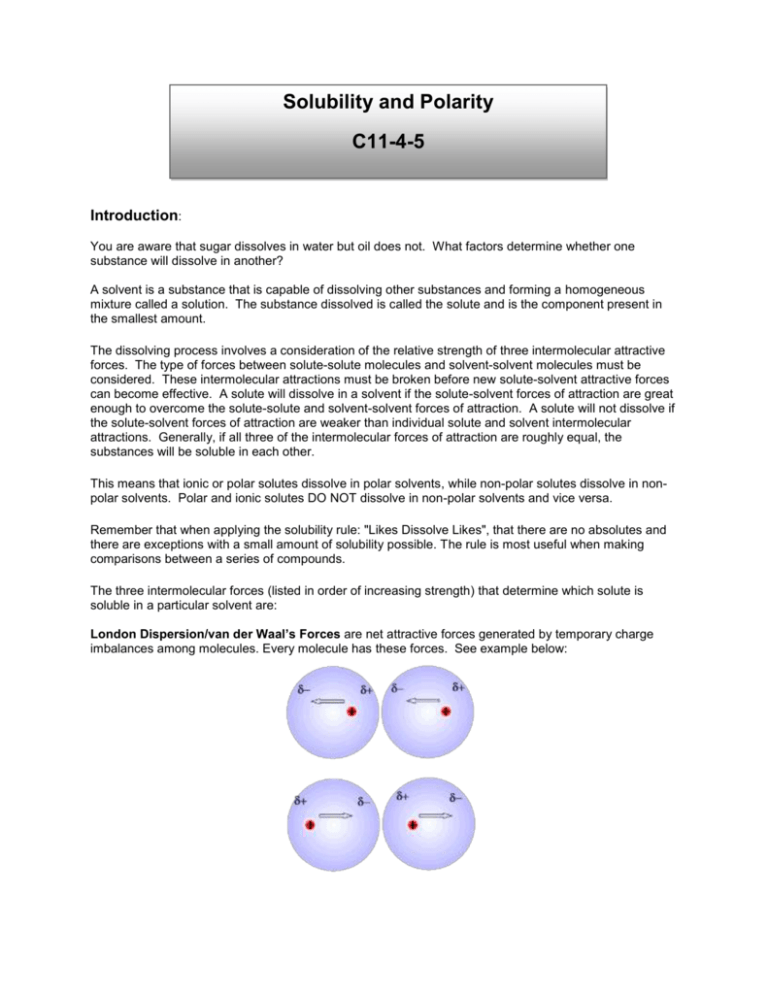
Comments
Post a Comment